12 Energetics & Redox Reactions
Introduction to Metabolism
Metabolism refers to the sum of chemical reactions that occur within a cell. Catabolism is the breakdown of organic and inorganic molecules, used to release energy and derive molecules that could be used for other reactions. Anabolism is the synthesis of more complex molecules from simpler organic and inorganic molecules, which requires energy.
Energetics
While some energy is lost as heat in chemical reactions, the measurement of interest for cells is the amount of free energy (G), or the energy available to do work. Cells perform three different types of work: chemical work (such as anabolism), transport work (such as nutrient uptake), and mechanical work (such as the rotation of a flagellum).
The change in free energy is typically denoted as ΔG°’, which indicates the change in free energy under standard conditions of pH 7, 25oC, 1 atmosphere pressure (also known as the standard free energy change). A reaction that generates a positive ΔG°’ indicates that the reaction requires energy and is endergonic in nature. A reaction that generates a negative ΔG°’ indicates that the reaction releases energy and is exergonic in nature. Reactions that are exergonic release energy that can be conserved by the cell to do work.
Adenosine triphosphate (ATP)
Adenosine triphosphate or ATP is a high-energy molecule used by all cells for energy currency, partly because it readily donates a phosphoryl group to other molecules. An exergonic reaction will release energy, driving the synthesis of ATP from the addition of a phosphate molecule (orthophosphate or Pi) to adenosine diphosphate or ADP. An endergonic reaction, which requires energy, will couple with the hydrolysis of ATP to ADP + Pi, using the released energy to drive the reaction.
Enzymes
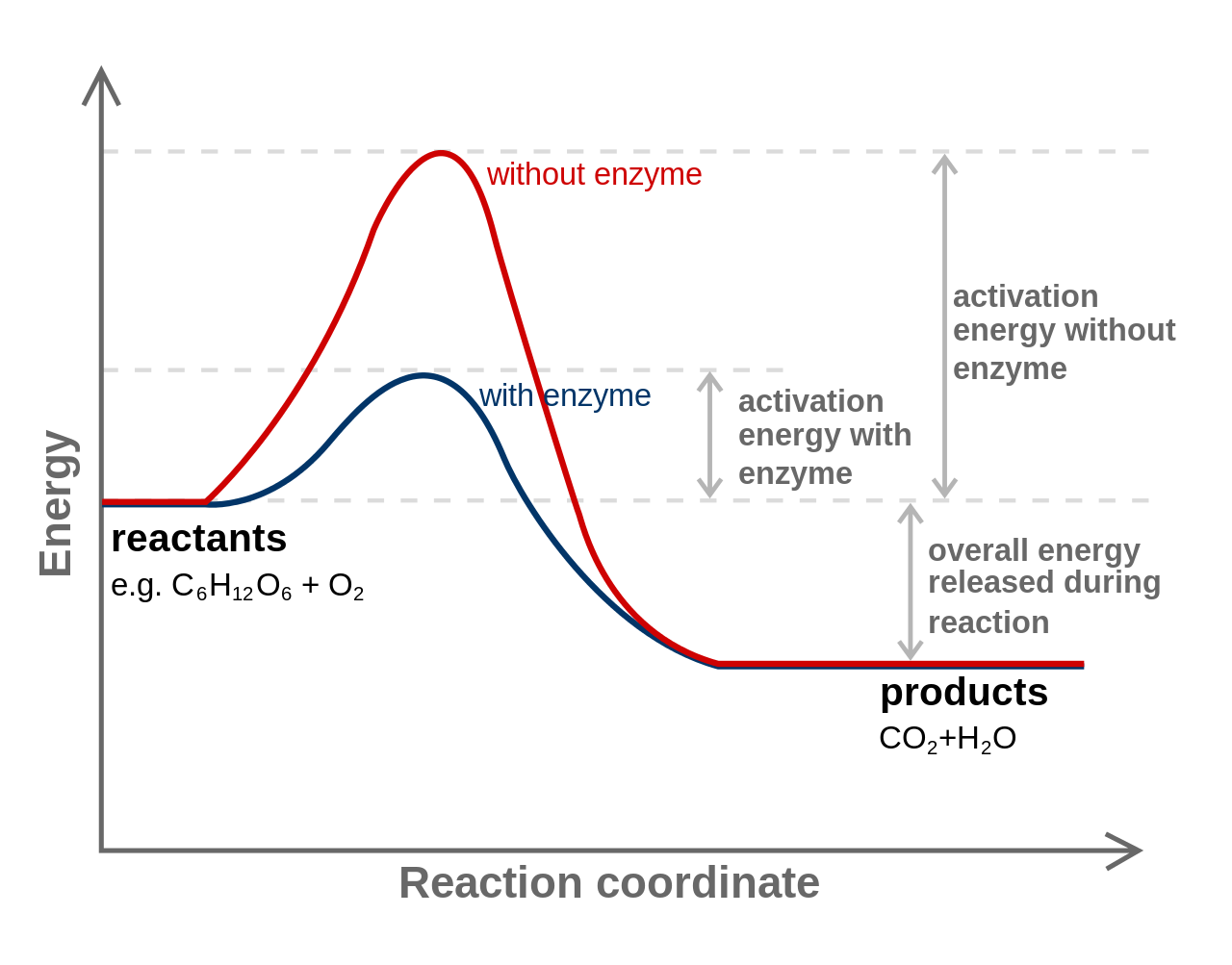
In order for a chemical reaction to proceed, chemical bonds must be broken. The energy required to break bonds is called activation energy. The amount of activation energy required by a cell can be lowered with the help of a catalyst, substances which assist the reaction to proceed without being changed themselves by the reaction. Cells use protein catalysts known as enzymes.
Redox Reactions
Cells conserve energy in the form of ATP by coupling its synthesis to the release of energy via oxidation-reduction (redox) reactions, where electrons are passed from an electron donor to an electron acceptor. The oxidation of a molecule refers to the loss of its electrons, while the reduction of a molecule refers to its gain of electrons. Organic chemists often refer to the process by the mnemonic OIL RIG: Oxidation Is Loss, Reduction Is Gain. A molecule being oxidized is acting as an electron donor, while the molecule being reduced is acting as an electron acceptor. Since electrons represent energy, a substance with many electrons to donate can be thought of as energy-rich.
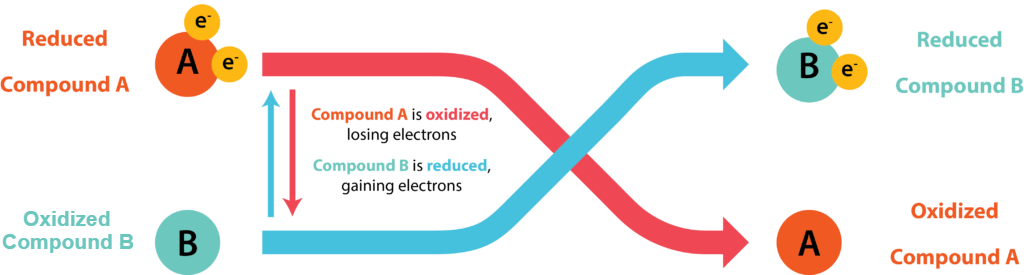
Electrons do not exist freely in solution, they must be coupled with atoms or molecules. Every redox reaction consists of two half reaction, where one substance donates electrons and thus becomes an oxidized product while another substance accepts the electrons and thus becomes a reduced product. Conjugate redox pair refers to the acceptor and donor of a half reaction.
Every redox reaction consists of two half reactions, where the reduced substance can donate electrons and thus become an oxidized product, while the oxidized substance can accept electrons and thus become a reduced product in a different reaction. A substance can be either an electron donor or an electron acceptor, dependent upon the other substances in the reaction.
An example would be ½ O2/H2O, where H2O could serve as an electron donor in one reaction, becoming the product O2 as a result of being oxidized, while O2 could serve as an electron acceptor in a different reaction, becoming the product H2O as a result of being reduced. In order for either reaction to be complete, another redox couple would need to participate
Each half reaction is given a standard reduction potential (E’0) in volts or millivolts, which is a measurement of the tendency of the donor in the reaction to give up electrons. A substance with greater tendency to donate electrons in the reduced form has a more negative E’0, while a substance with a weak tendency to donate electrons in the reduced form has a less negative or even positive E’0. A substance with a negative E’0 makes a very good electron donor, in the reduced form.
Redox Tower
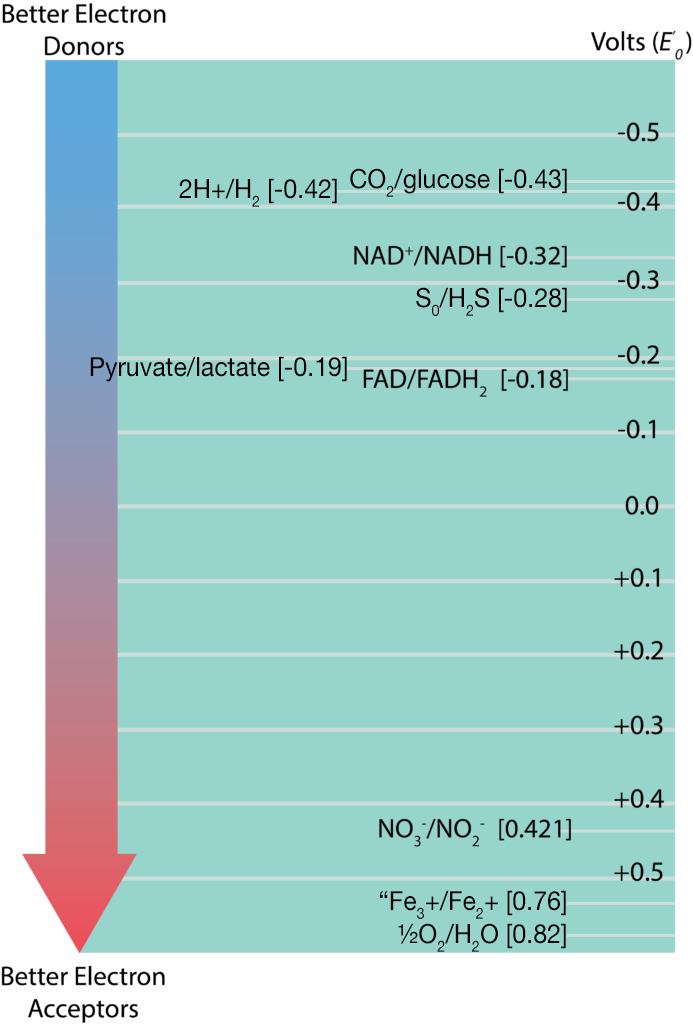
The information regarding standard reduction potentials for various redox couples is displayed in the form of a redox tower, which lists the couples in a vertical form based on their E’0. Redox couples with the most negative E’0 on listed at the top while those with the most positive E’0 are listed on the bottom. The reduced substance with the greatest tendency to donate electrons would be found at the top of the tower on the right, while the oxidized substance with the greatest tendency to accept electrons would be found at the bottom of the tower on the left. Redox couples in the middle can serve as either electron donors or acceptors, depending upon what substance they partner with for a reaction. Applying this information to the redox couples shown in the electron tower below, the best initial electron donor would be glucose, while the best electron acceptor would be oxygen. This would result in the products CO2 and H20 being formed.
The difference between reduction potentials of a donor and an acceptor (ΔE’0) is measured as acceptor E’0 minus donor E’0. The larger the value for ΔE’0, the more potential energy for a cell. Larger values are derived when there is the biggest distance between the donor and the acceptor (or a bigger fall down the tower).
While ΔE’0 is proportional to ΔG°’, the number of electrons that a substance has to donate is important too. The actual formula is:
[latex]\Delta \mathrm{G}^{\circ\prime} = -nF \cdot \Delta {\mathrm{E}^{\prime}}_{0}[/latex]
where n is the number of electrons being transferred and F is the Faraday constant (23,062 cal/mole-volt, 96, 480 J/mole-volt).
Electron Carriers
The transference of electrons from donor to acceptor does not occur directly, since chemically dissimilar electron donors and acceptors might never interact with one another. Instead, many cellular intermediates participate in the process, with the possibility for energy capture occurring along the way. These intermediates are called electron carriers and they go back and forth between a reduced form (when they are carrying an electron) and an oxidized form (after they have passed the electron on), without being consumed in the reaction themselves. It is important to note that electron carriers can never serve as the initial electron donor or the final electron acceptor for a reaction, since they originate within the cell itself and need to be constantly recycled in order to continually participate in reactions. The cell needs to rely upon external chemicals that it transports in to act as the initial electron donor and the final electron acceptor. Products that are formed as a result of the reaction (i.e. the now oxidized electron donor and the now reduced electron acceptor) are often waste products for the cell and can be released to the environment.
In order for the reaction to be energetically favorable for the cell, the carriers must be arranged in order of their standard reduction potential (i.e. going down the redox tower), with an electron being passed from a carrier with the most negative E’0 to a carrier with a less negative E’0. It is important to note that some carriers accept both electrons and protons, while other carriers accept electrons only. This fact will become of crucial importance later, in the discussion of how energy is generated.
While there are many different electron carriers, some unique to specific organisms or groups of organisms, let us cover some of the more common ones:
- Nicotinamide adenine dinucleotide (NAD+/NADH) – a co-enzyme that carriers both electrons (e-) and protons (H+), two of each. A closely related molecule is nicotinamide adenine dinucleotide phosphate (NADP+/ NADPH), which accepts 2 electrons and 1 proton.
- Flavin adenine dinucleotide (FAD/FADH) and flavin mononucleotide (FMN/FMNH) – carry 2 electrons and 2 protons each. Proteins with these molecules are called flavoproteins.
- Coenzyme Q (CoQ)/ubiquinone – carries 2 electrons and 2 protons.
- Cytochromes – use iron atoms as part of a heme group to carry 1 electron at a time.
- Iron-sulfur (Fe-S) proteins, such as ferredoxin – use iron atoms not part of heme group to carry 1 electron at a time.
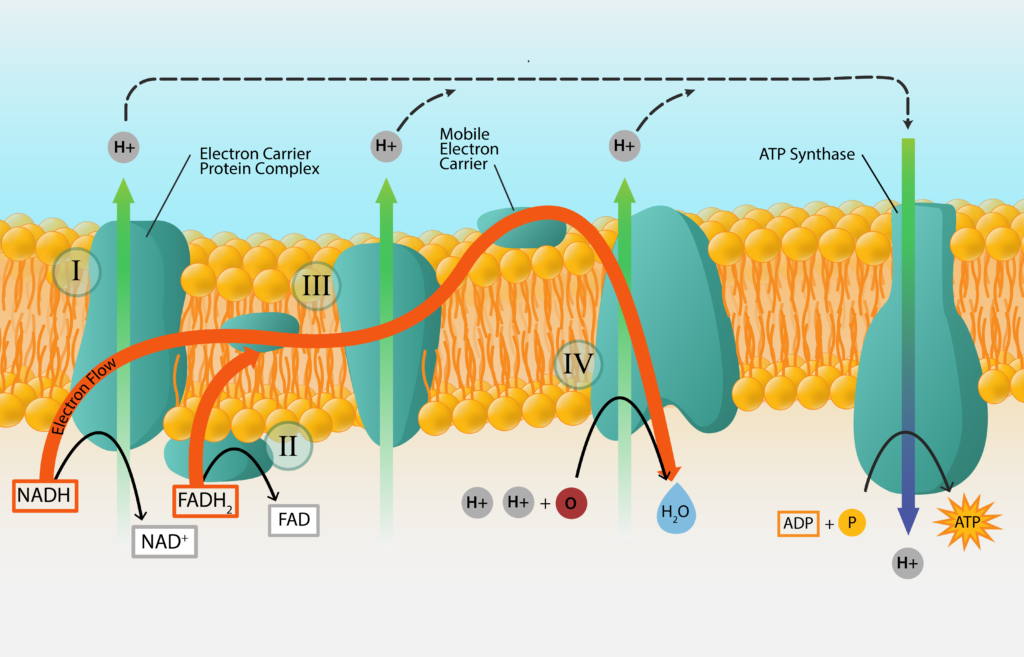
Electron Transport Chain
The process starts with an initial electron donor, a substance from outside of the cell, and ends with a final electron acceptor, another substance from outside of the cell. In the middle the electrons are passed from carrier to carrier, as the electrons work their way down the electron tower. In order to make the process more efficient, most of the electron carriers are embedded within a membrane of the cell, in the order that they are arranged on a redox tower. These electron transport chains are found within the cell membrane of bacteria and archaea, and within the mitochondrial membrane of eukaryotes.
Key Words
metabolism, catabolism, anabolism, free energy (G), chemical work, transport work, mechanical work, ΔG°’, standard free energy change, exergonic, endergonic, adenosine triphosphate (ATP), orthophosphate (Pi), activation energy, catalyst, enzyme, oxidation-reduction (redox) reaction, electron donor, electron acceptor, OIL RIG, conjugate redox pair, redox couple, standard reduction potential (E’0), redox tower, ΔE’0, electron carriers, nicotinamide adenine dinucleotide (NAD+/NADH), nicotinamide adenine dinucleotide phosphate (NADP+/ NADPH), flavin adenine dinucleotide (FAD/FADH), flavin mononucleotide (FMN/FMNH), coenzyme Q (CoQ)/ubiquinone, cytochrome, iron-sulfur (Fe-S) proteins, ferredoxin, electron transport chain (ETC).
Study Questions
- How are metabolism, catabolism, and anabolism defined?
- What are the 3 major types of work carried out by cells? What’s an example of each type?
- What is free energy? What is standard free energy?
- What are the characteristics of an endergonic and an exergonic reaction? How can cells conserve the energy given off by reactions?
- What is the role of ATP in the cell and why it is a good compound for this role?
- What are enzymes? What role do enzymes play in energy conservation?
- What is oxidation and reduction? What does standard reduction potential represent, such as 2H+/H2 = -0.42V? Indicate what each term in this equation represents. In redox pairs with a more negative O-R potential the reduced form is more likely to be an electron ____________________ and has __________________________ potential energy. What is a conjugate redox pair?
- What is an electron tower and how does this concept help to explain energy exchange in a cell?
- What is ΔG0’? What does it represent and how is it calculated?
- What is an electron carrier, what role do they play, what are the most common electron carriers in the cell and why must they constantly be recycled?
- What is an electron transport chain and how does it function to conserve energy for the cell?

