5. Carbon
Due to the importance of CO2 as a greenhouse gas the carbon cycle is a crucial part of the climate system. Since carbon exchanges with the biosphere, biological processes need to be considered in climate science. The carbon cycle is part of the broader biogeochemical cycles, which include other biologically important chemical elements such as nitrogen and oxygen.
a) The Natural Carbon Cycle
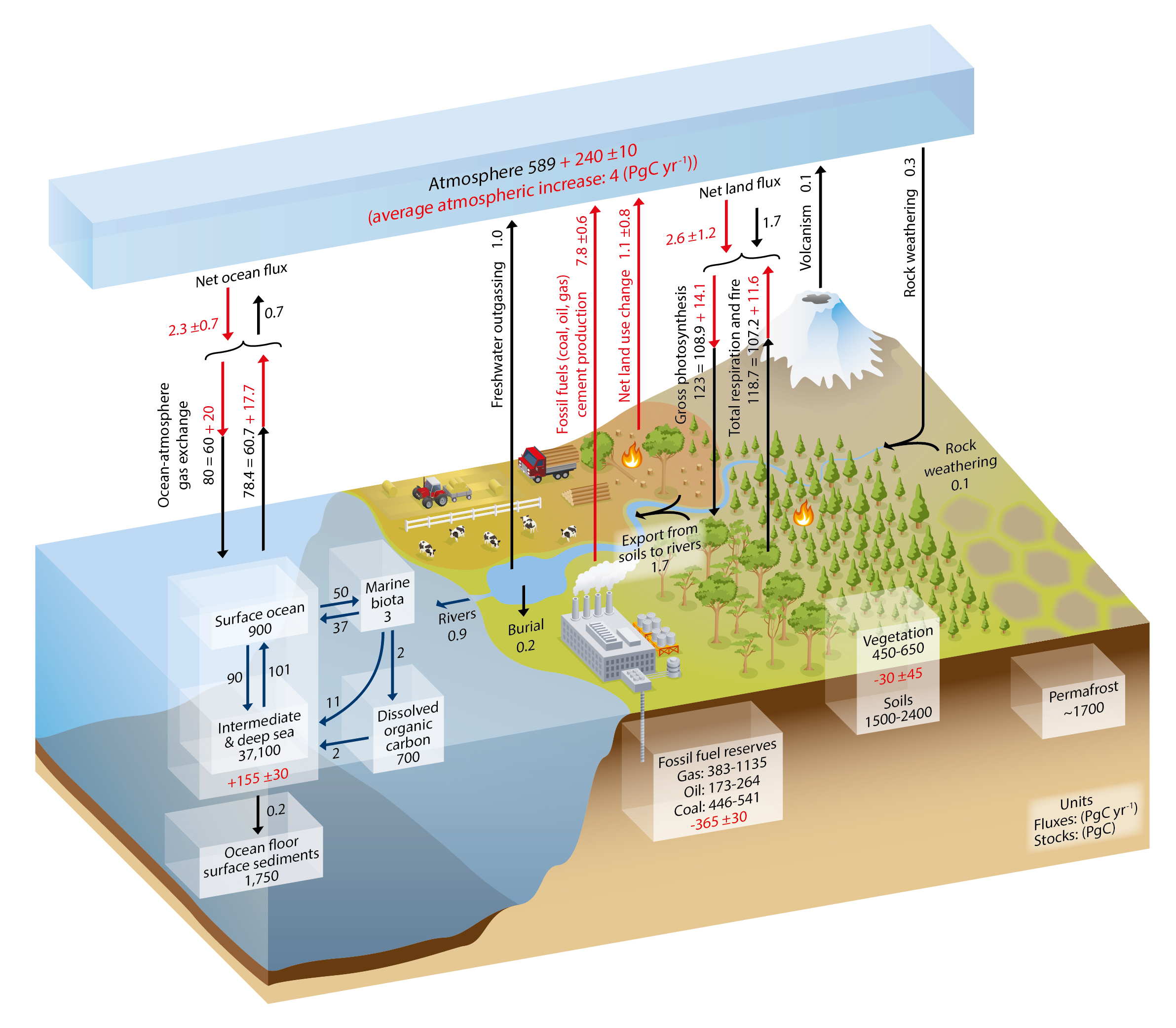
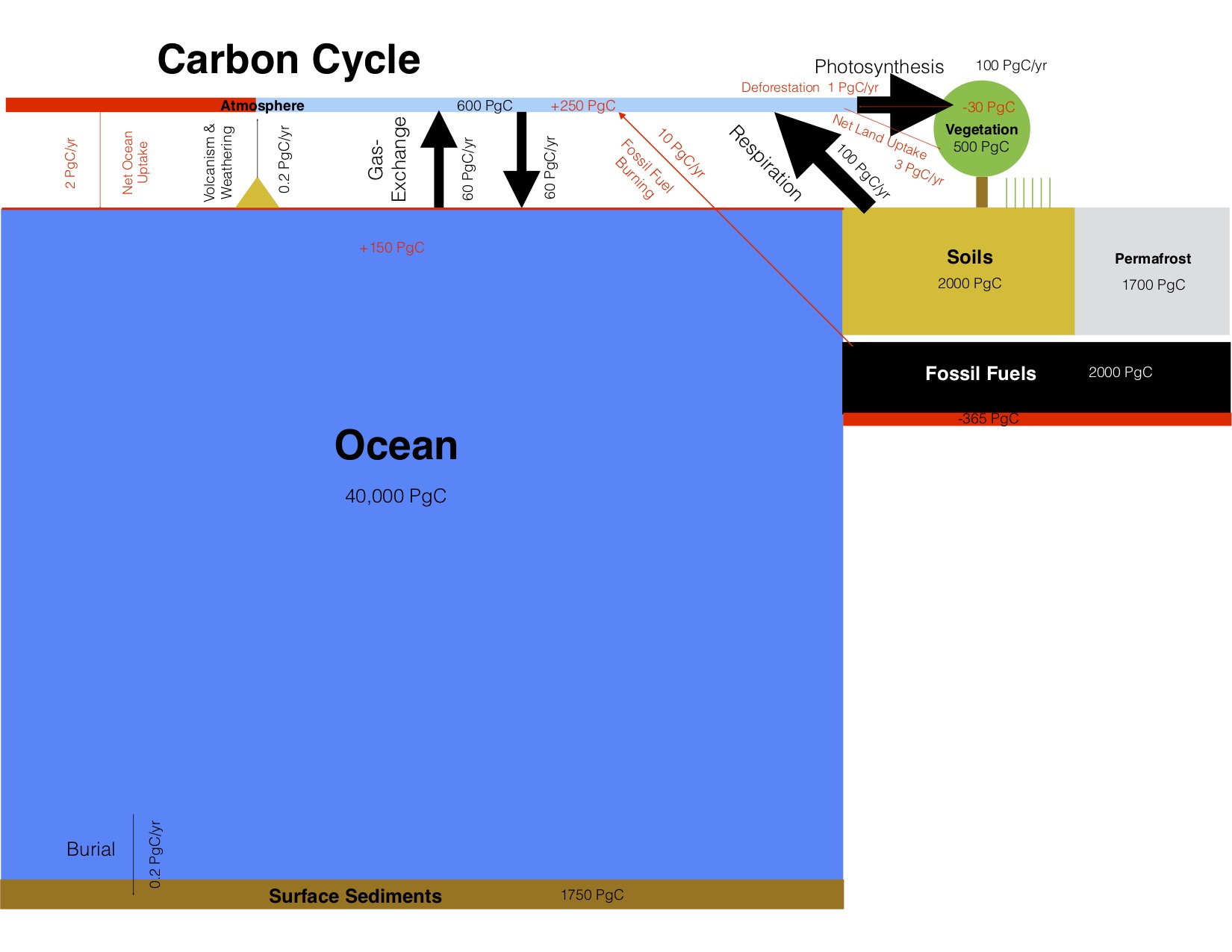
Carbon exchanges relatively rapidly between three large reservoirs: the ocean, the atmosphere, and the land (Fig. 1). Of those the ocean contains the most carbon: almost 39,000 Pg (37,100+900+700+3). Most of the carbon in the ocean is in the form of Dissolved Inorganic Carbon (DIC), and most DIC resides in the intermediate and deep layers because those depths make up most of its volume. Marine biota are important in transferring carbon from the surface to the deep ocean, but their biomass is very small because they consist mainly of microscopic algae called phytoplankton. Phytoplankton build the base of the ocean’s food web through photosynthesis. They have adapted to be tiny and light, so as not to sink to the sea floor. They need to stay near the sunlit surface to photosynthesize. Below about 100 m depth light levels get too low due to absorption of sunlight by sea water. The deep ocean is therefore dark but organic matter sinks there from the surface in various forms, e.g. as fecal pellets of zooplankton. Below the surface, the sinking dead organic matter is respired by bacteria and returned into the inorganic carbon pool. This is called the biological pump because it removes carbon from the surface and atmosphere and sequesters it in the deep ocean, where it can stay for hundreds to thousands of years. Dissolved CO2 gas in sea water is part of the DIC pool. It exchanges with the atmosphere more than 50 Pg of carbon per year. Ocean-atmosphere gas exchange depends on the difference between surface ocean and atmospheric partial pressures (pCO2; in this book we use pCO2 and CO2 concentration synonymously in units of parts per million or ppm) and therefore leads to a strong and relatively rapid coupling of the atmospheric CO2 concentration to the surface ocean.
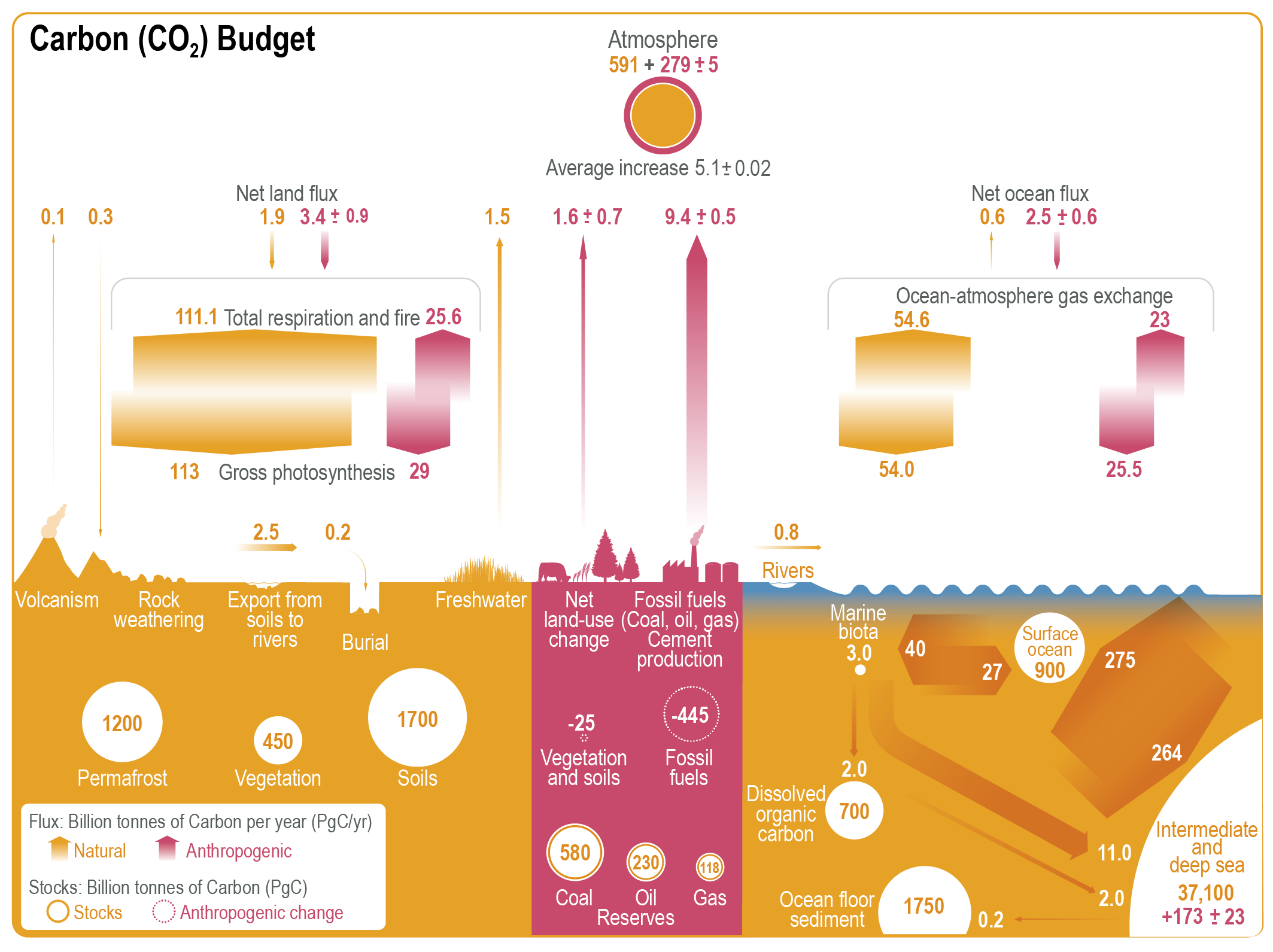
The second biggest of the three rapidly exchanging carbon reservoirs is the land, which contains more than 3,000 Pg of carbon. On land carbon is stored in living vegetation, in soils, and in permafrost. Since land plants don’t have the problem of sinking out of the light they can grow big and contain large amounts of carbon, such as trees. Therefore, much more carbon is stored in living biomass on land (~450 Pg) than in the ocean (~3 Pg). However, even more carbon is stored in soils and permafrost.
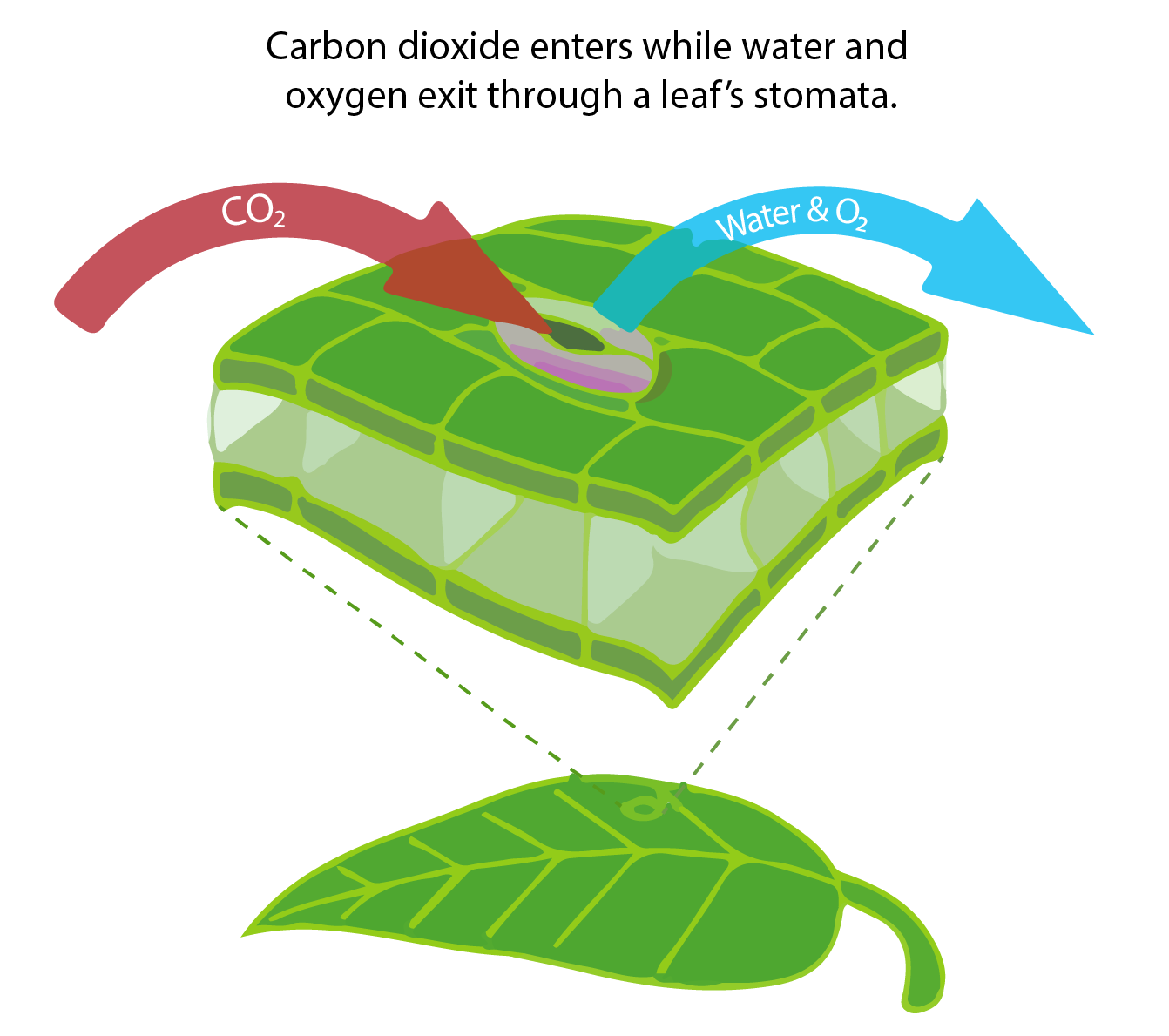
More than 100 Pg of carbon are removed from the atmosphere each year by photosynthesis of land plants and turned into organic matter. Organic matter cycles through the land food web and eventually gets into the soil carbon pool where it decomposes. Like in the ocean, bacteria and heterotrophic organisms on land respire organic carbon and turn it back into inorganic CO2. Land carbon uptake and release does not depend strongly on atmospheric CO2 concentrations; water availability and temperature, respectively, are more important. Plant growth on land is strongly water limited and respiration rates strongly depend on temperature. However, CO2 increases the water use efficiency of land plants because at higher CO2 concentrations they don’t need to open the stomata as much as at lower CO2 concentrations. Stomata are small openings in the cells that allow CO2 to enter, but they also allow water to leave in a process called transpiration (see figure in box below). Thus, at higher CO2 levels plants can grow more for the same amount of water usage.
The atmospheric carbon reservoir is relatively small compared to the ocean, which is ~40 times bigger, and the land, which is ~10 times bigger. However, the atmosphere is crucial in linking land and ocean through rapid exchanges.
Relatively small amounts of carbon from land enter the ocean via rivers (0.8 Pg C/yr). Exchanges between the lithosphere and the ocean-atmosphere-land system are associated with the slow accumulation of carbon in sea floor sediments (0.2 Pg C/yr), CO2 emissions from vulcanism (0.1 Pg C/yr), and rock weathering, which removes 0.1 PgC/yr from the lithosphere (in addition to the 0.3 Pg C/yr it removes from the atmosphere).
Box 1: Photosynthesis and Respiration
Photosynthesis (Fig. B1.1) is the process by which autotrophic organisms (plants, algae, and many bacteria) produce organic matter and oxygen from CO2 and water using light as an energy source.

Respiration (Fig. B1.2) is the reverse process by which heterotrophic organisms (bacteria, fungi, animals, and humans) oxidize organic carbohydrates to derive their energy resulting in CO2 and water.
In order to photosynthesize, land plants have to take up CO2 from the air. They do this by opening little pores called stomata, through which not only CO2 can enter, but also water and oxygen can leave the cell (Fig. B1.3).
b) Anthropogenic Carbon
Human effects on the global carbon cycle have been relatively limited before the industrial revolution, although some emissions from land-use change such as deforestation may have been going on for hundreds or thousands of years. During the last 100 years or so, however, rapid burning of fossil fuels such as coal, gas, and oil have caused a massive perturbation of the carbon cycle (Fig. 2). This perturbation is perhaps most evident in the atmosphere where CO2 concentrations have increased by more than 40 %. In chapter 3 we have seen that current levels of atmospheric CO2 are unprecedented during the last 800,000 years, but reconstructions going back further in time indicate that the last time Earth’s atmosphere had about 400 ppm CO2 was about 3 million years ago.
Currently humans emit more than 10 billion metric tons of carbon into the atmosphere per year mostly from fossil fuel burning (~85 %), although deforestation continues to be a significant contribution (~15 %). Anthropogenic carbon emissions from fossil fuel burning have increased rapidly after World War Two. The ocean takes up about 23 % (2.5/(9.4+1.6); Fig. 1) of current anthropogenic emissions, while the land takes up 31 % (3.4/11). Historically, the ocean has absorbed about 37 % (173/(445+25)) of all anthropogenic carbon emissions hitherto, whereas 59 % (279/470) have stayed in the atmosphere. Land carbon has decreased only slightly (-5 %) because of compensating effects due to loss from deforestation and gain from recent vegetation regrowth.
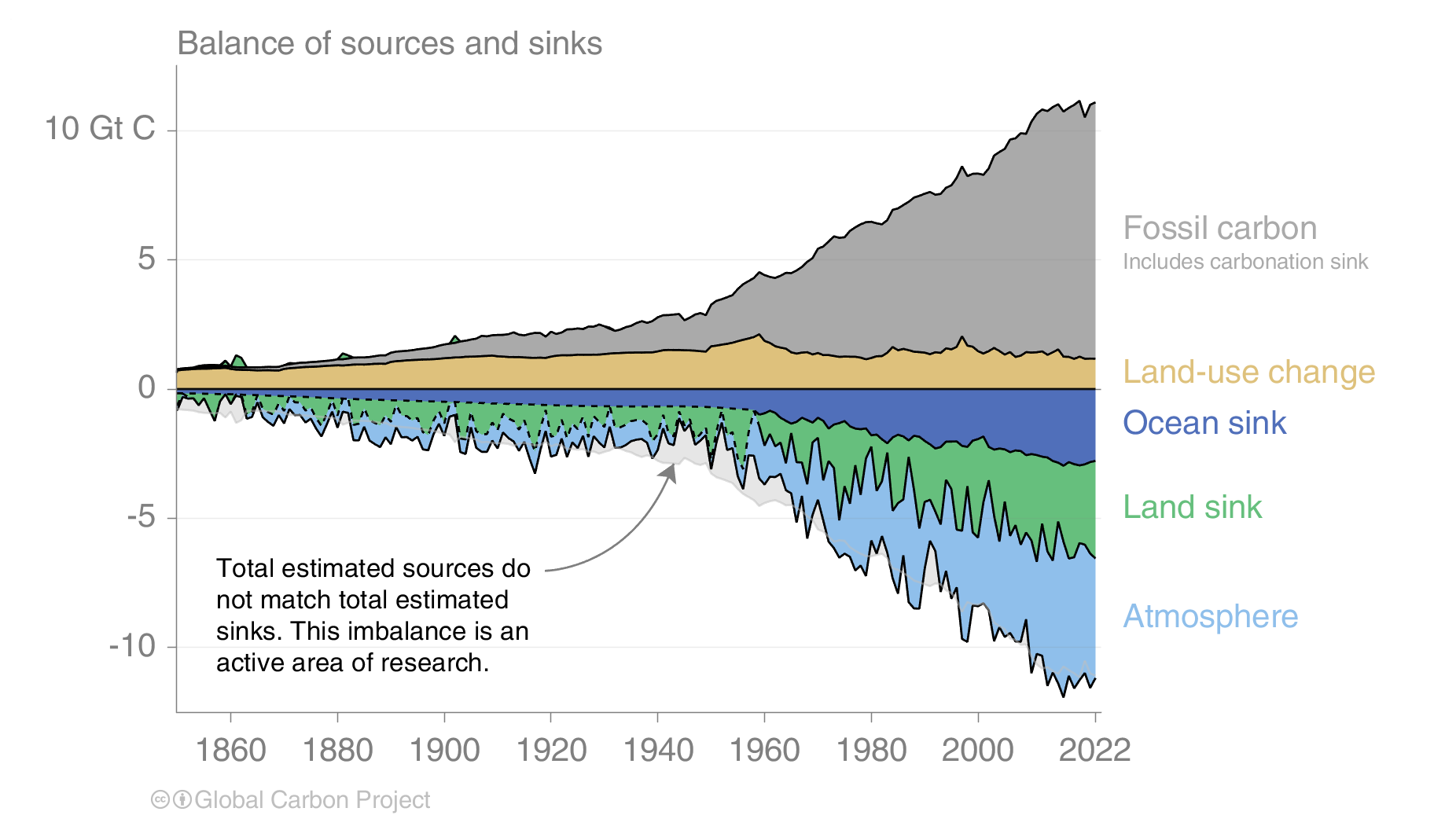
Anthropogenic carbon emissions have increased rapidly over the past 60 years but in recent years they have flattened out (Fig. 3). About half of all carbon put into the atmosphere by humans since the industrial revolution (cumulative emissions) was done so in the last 30 years. Cumulative emissions are the grey and brown areas in Fig. 2. Together they amount to about 500 GtC or half a trillion metric tons. As we will see later cumulative carbon emissions determine the global temperature increase.
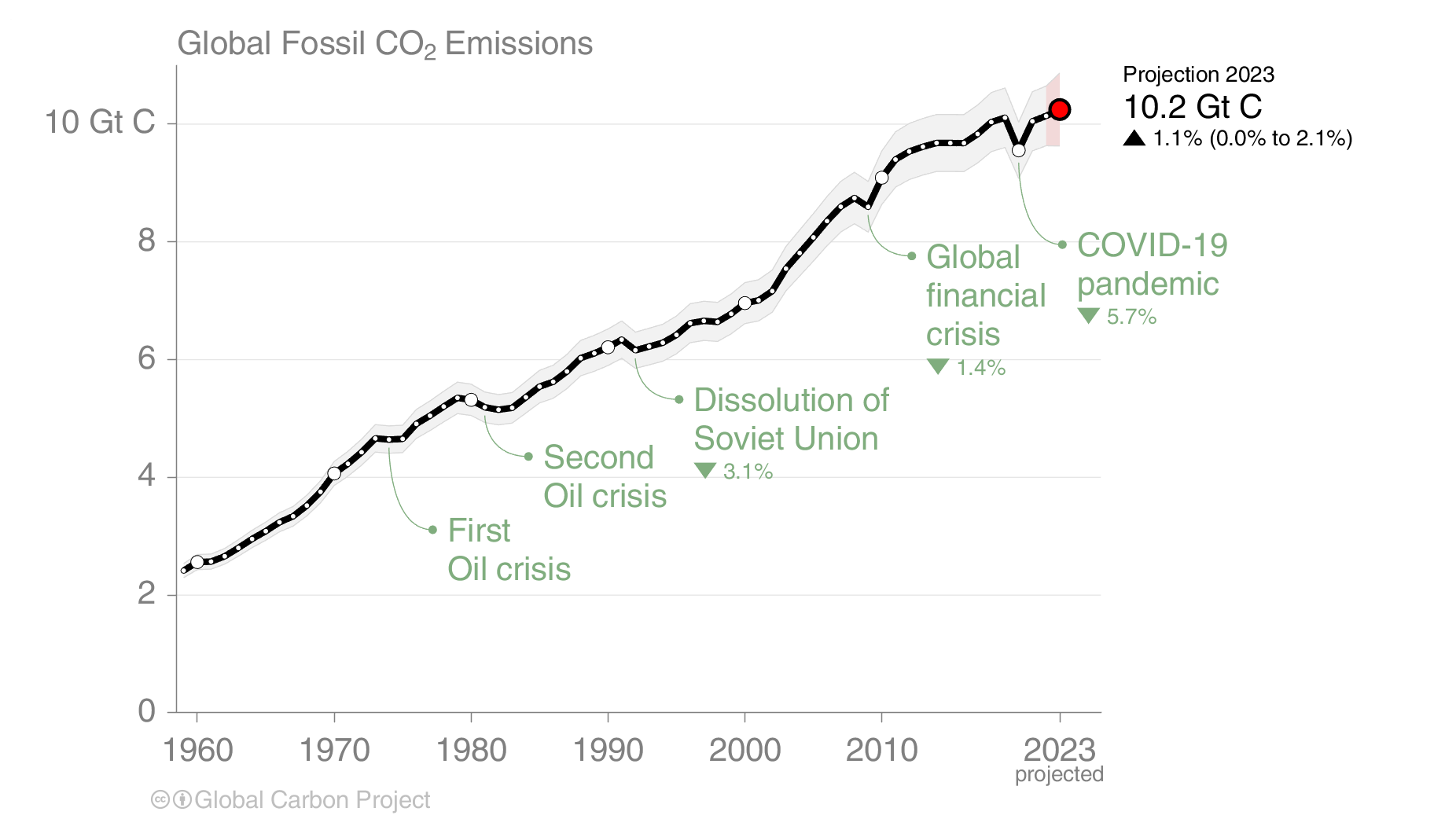
The effects of the financial and economic crisis is seen in the dip in global carbon emissions in 2009 caused by emission reductions in the US and Europe (Fig. 4), whereas emissions continued to increase in China until 2013 after which they stayed constant. A similar dip occured during the economic slowdown associated with the coronavirus pandemic.
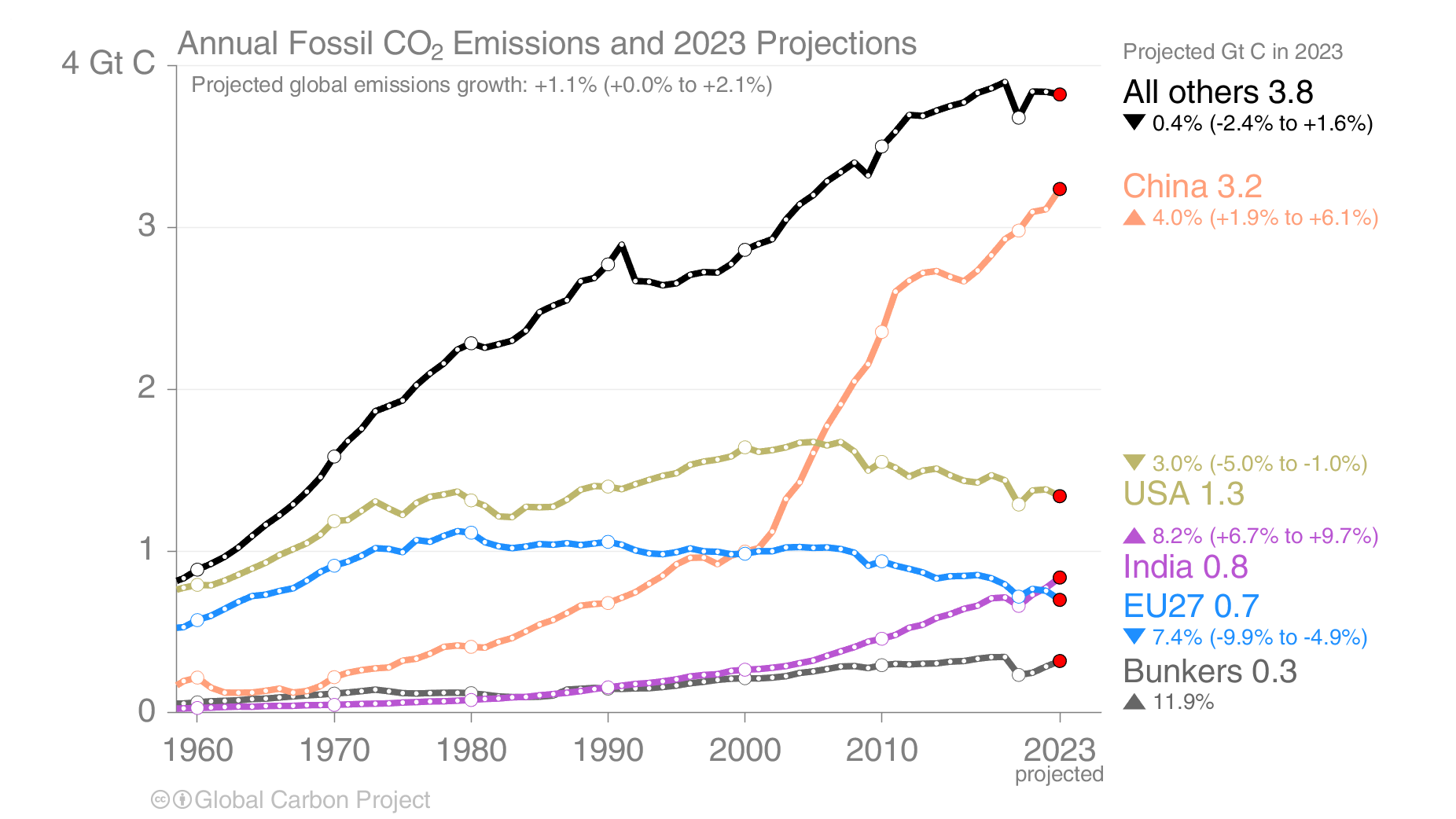
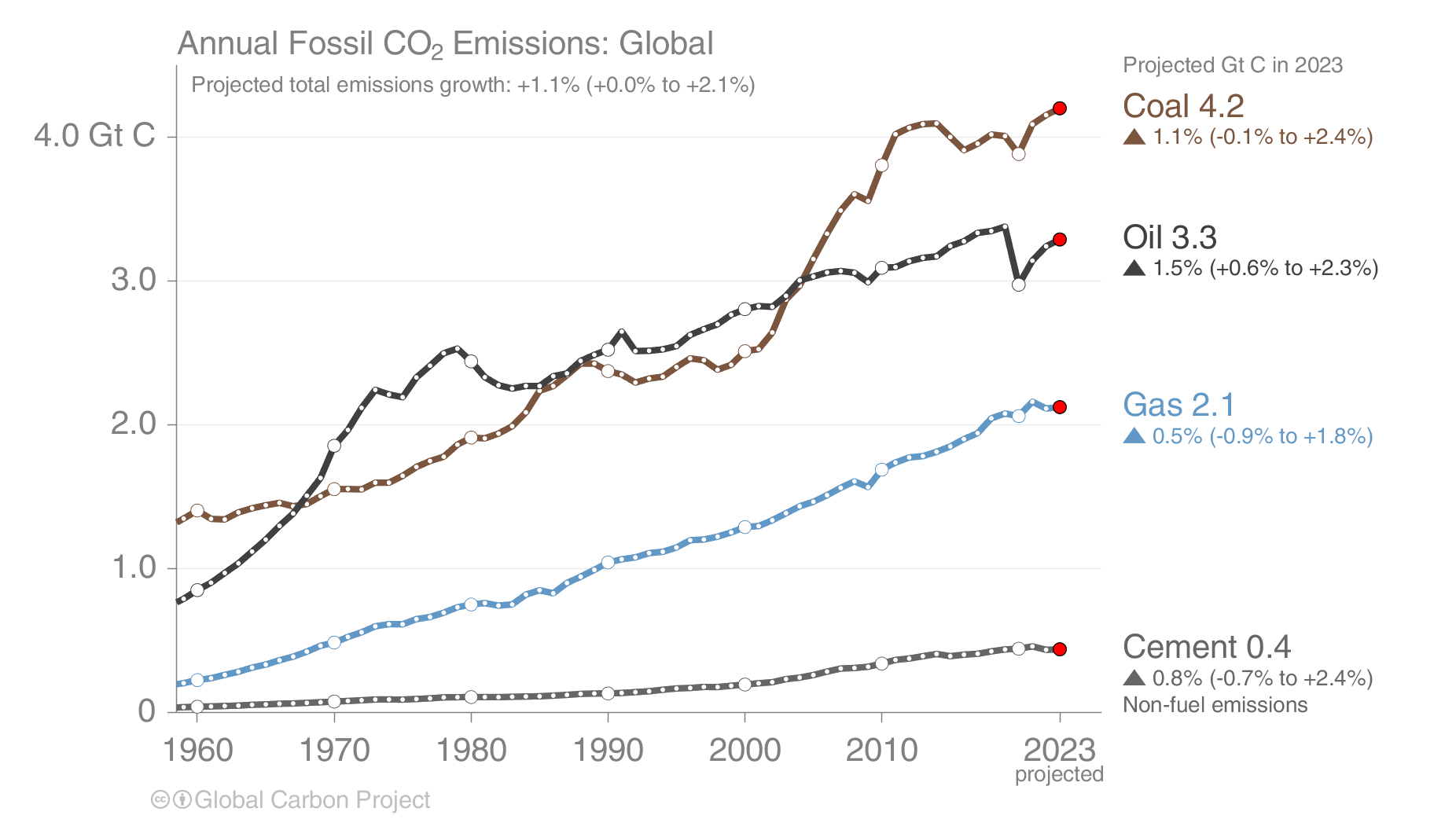
Human caused carbon emissions are mainly from burning of fossil fuels, whereas cement production contributes only about 6 % (Fig. 5). Burning of coal, oil, and gas have all increased substantially during the last 50 years. The increase in emissions from China during the first decade of the 21st century was fueled mainly by coal burning.
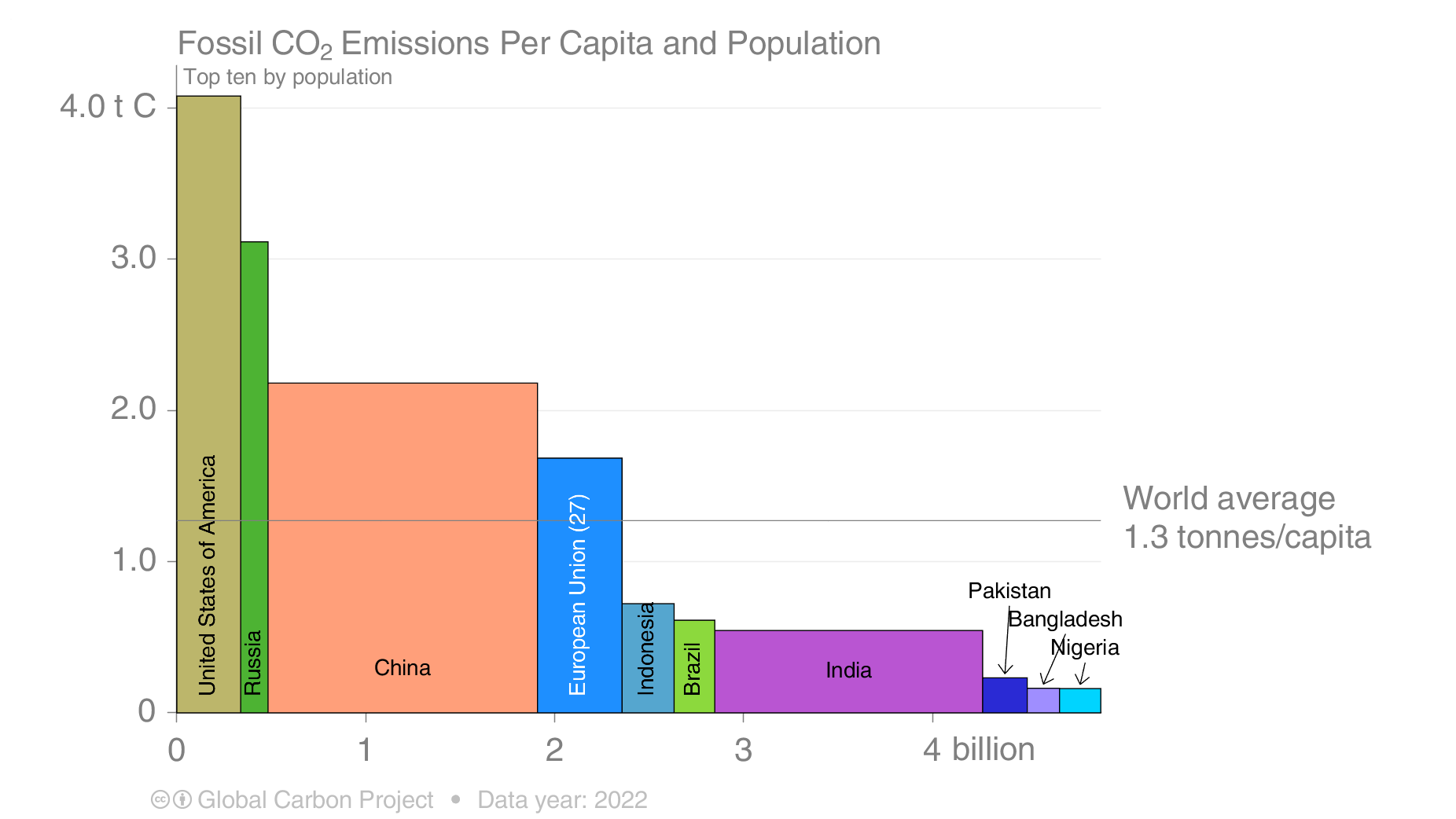
Among the four top emitters the US is the one with the largest emissions per person (Fig. 6). The average US American emits more than 4 metric tons of carbon into the air each year. This is more than twice the emissions per person in Europe or China, more than three times the average emissions world-wide, and about ten times the emissions from a person in India. The US is responsible for 25% of all carbon emitted in the past (cumulative emissions) although it makes up only 4% of the world’s population. Europe, which accounts for about 10% or the world’s population, has emitted more than 22% of all carbon. This figure shows other countries.
How do we know that the rising CO2 concentrations in the atmosphere are from human activities? There are several independent lines of evidence. The first comes from economic data. Since fossil fuels are traded internationally we know how much oil, coal, and gas a country imports and uses. The data shown in Figs. (2) through (6) are based on these estimates. The shaded area in Fig. (3) indicates the error bars of those estimates. Not all countries publish and make their data available, which leads to these uncertainties. However, they are relatively small such that the emissions are known to within about a 5 % error margin.
Box 2: Carbon Isotopes
Carbon exists as three isotopes. The most common carbon-12 (12C) with 6 protons and 6 neutrons, the rarer carbon-13 (13C) with an additional neutron, and carbon-14 (14C) or radiocarbon with two additional neutrons. 14C is radioactive and decays with a half-life of 5,730 years.
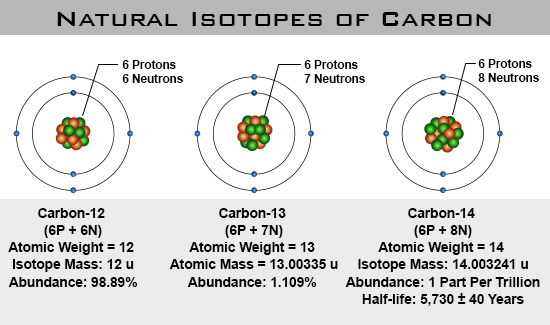
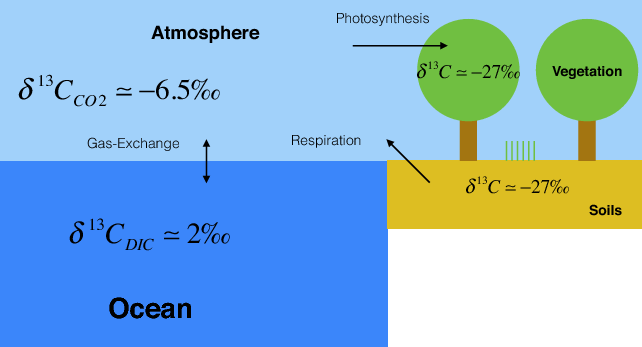
Plants and algae fractionate carbon isotopes by about 20 ‰ during photosynthesis such that they preferentially take up the light 12C. Pre-industrial δ13C values of atmospheric CO2 were about -6.5 ‰. Thus plant and soil carbon have δ13C values of around -27 ‰.
The delta notation is analogous to that of oxygen isotopes discussed in chapter 3. δ13C = R/Rstd – 1, where R = 13C/12C is the ratio of the heavy over the light isotope and Rstd is that of a standard.
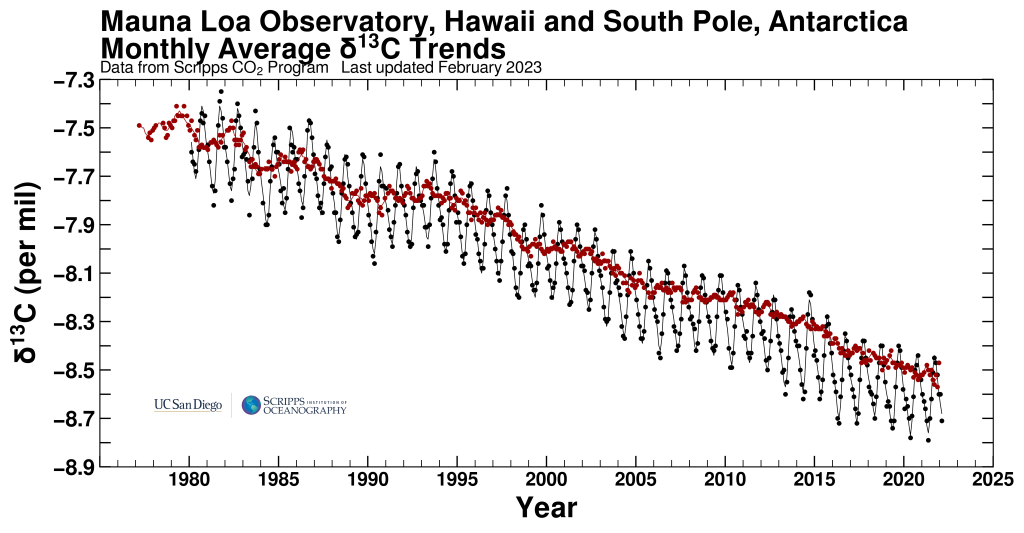
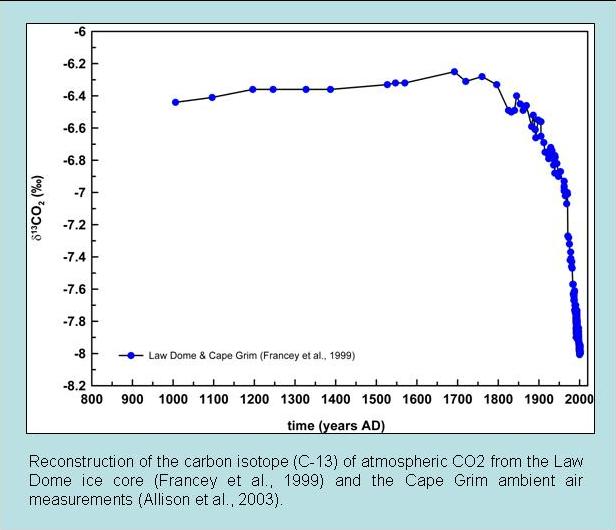
The second line of evidence is based on carbon isotope measurements. Fractionation during photosynthesis leads to plants and algae having very depleted δ13C values (see box Carbon Isotopes). Since fossil fuels are derived from ancient plants they are depleted in 13C isotopes as well. Thus the addition of carbon with a very depleted 13C signature to the atmosphere leads to a decrease in δ13C values of atmospheric CO2. This is observed in measurements both from ambient air and air extracted from ice cores (Fig. 7).
The third line of evidence is based on oxygen measurements in air. Burning of fossil fuels has a similar chemical reaction equation that that of respiration. Carbohydrates react with oxygen to form CO2 and water. Energy is released during this reaction. Thus, burning of carbohydrates consumes oxygen. By measuring the oxygen to nitrogen ratio in air the changes in atmospheric oxygen concentration can be detected even though they are small compared to the absolute oxygen concentrations (Fig. 8). These measurements are evidence of a massive combustion process happening on Earth right now.
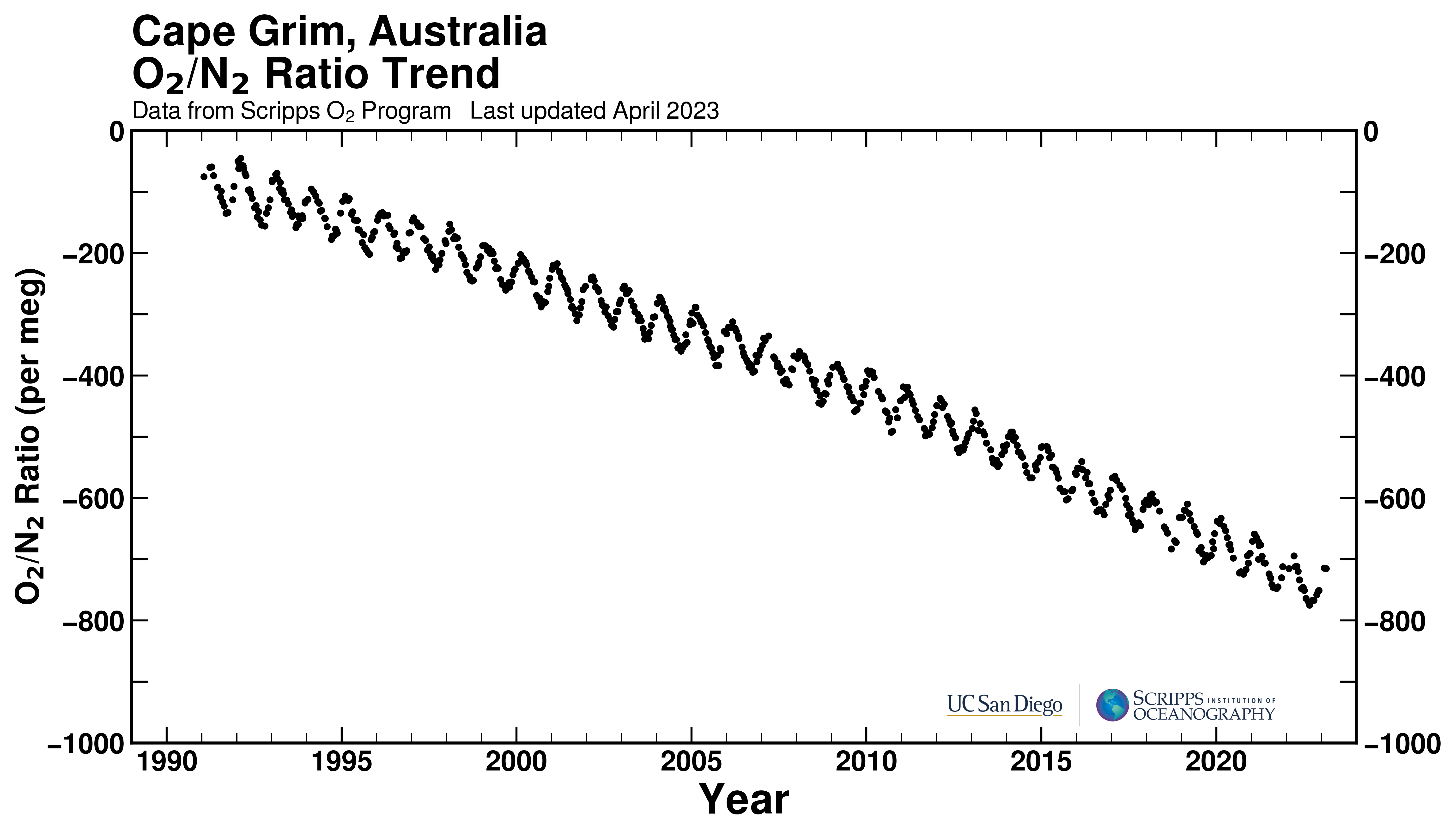
We conclude that humans have caused a large perturbation to the natural carbon cycle mostly by the burning of fossil fuels, which has increased atmospheric CO2 concentrations from 280 ppm to more than 400 ppm, to levels unprecedented in Earth’s history for about 3 million years. About 40 % of the anthropogenic carbon emitted so far has been taken up by the ocean, thus reducing the accumulation of CO2 in the atmosphere.
Box 3: Residence Time
The residence time τ of a substance in a reservoir is the time required to completely replace the reservoir with its input: τ = X/I = X/O (at equilibrium I = O), where X is the reservoir size (a.k.a. stock, amount, or inventory) and I (O) is the input (output) flux. See Budget Equation Box in Chapter 4.
Exercise: Use Fig. 1 to calculate
- for the pre-industrial period the residence times of carbon (tip: sum up all the inputs or outputs to calculate I or O) in the
- atmosphere,
- ocean,
- land,
- combined ocean-atmosphere-land system, and
- the residence time of anthropogenic carbon in the combined ocean-atmosphere-land system.
c) Carbonate Chemistry and Ocean Acidification
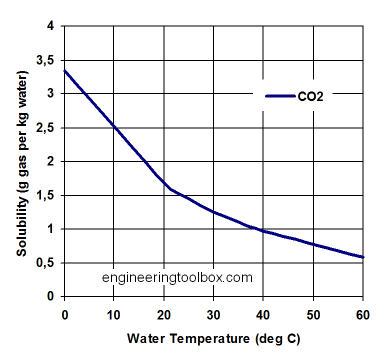
CO2 enters the ocean from the atmosphere through gas exchange if the partial pressure in the atmosphere is larger than the partial pressure in the ocean. It dissolves as CO2 gas in water just like it is dissolved in your soda drink. If you look at an unopened bottle of soda, you do not see bubbles. The CO2 molecules are emerged within a vast number of water molecules. The drink was bottled under pressure or under cold temperatures. Solubility of CO2 like that of other gases such as oxygen depends on temperature. More gas can be dissolved in colder water (Fig. 9). This is the reason why as you warm up a soda drink it will lose CO2. Because it is not liquid, CO2 does not ‘evaporate’ into the air, but it outgasses. Evaporation implies a phase change, which does not occur in this case.
In the ocean CO2 reacts with seawater to form carbonic acid (H2CO3), which dissociates into bicarbonate (HCO3–) and carbonate (CO32-) ions:
(1) ![]()
The sum of these three carbon species is called dissolved inorganic carbon (DIC = CO2 + HCO3– + CO32-) or total carbon. The equilibrium between the species depends on the pH. In the current ocean, pH is about 8.1, which leads to about 86.5 % of DIC being in the form of bicarbonate ions, 13.0 % in the form of carbonate ions, and only 0.5 % in the form of aqueous CO2 (Fig. 10; Zeebe and Wolf-Gladrow, 2001).
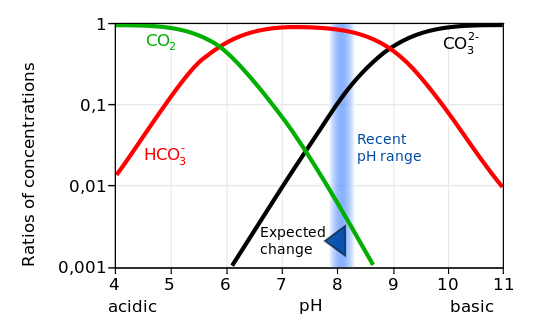
Dissociation of carbonic acid into bicarbonate (baking soda) produces a hydrogen ion H+, which decreases the pH of the water. Most hydrogen ions, however, re-combine with carbonate ions to form additional bicarbonate ions. Nevertheless, adding CO2 to seawater increases its hydrogen ion concentration (decreases its pH) and decreases the carbonate ion concentration. This process is called ocean acidification.
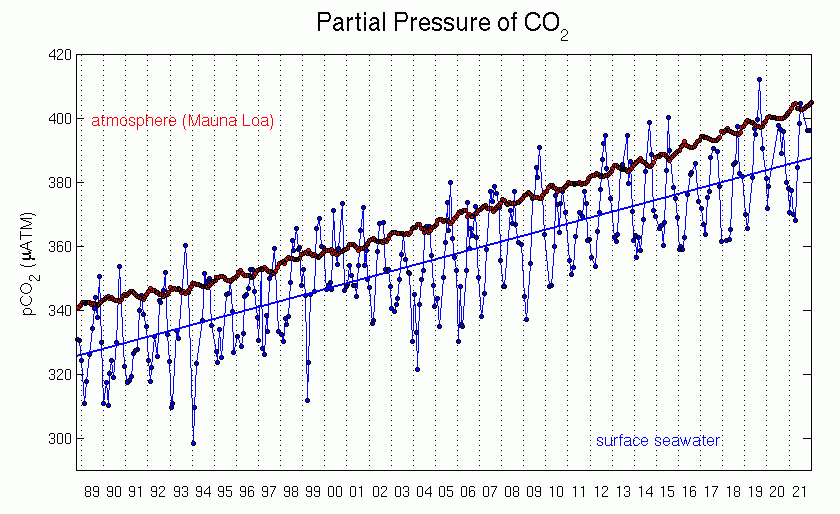
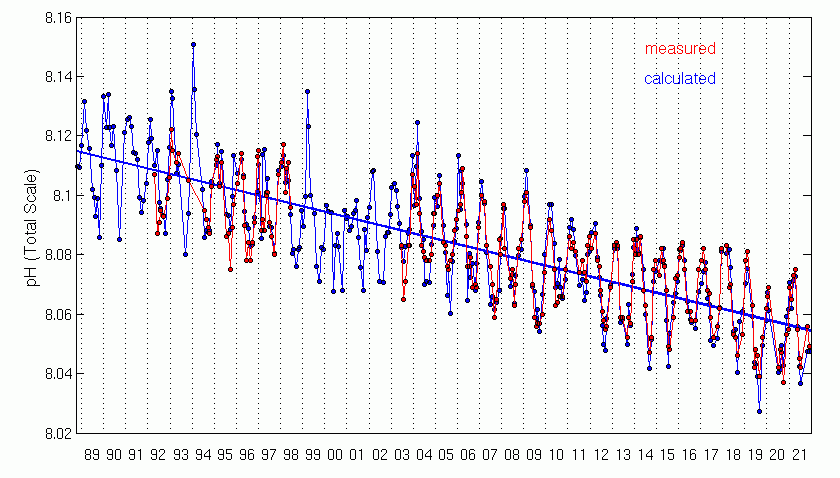
Observations show that the partial pressure of surface ocean water follows closely the trend in atmospheric CO2 (Fig. 11) indicating the uptake of anthropogenic carbon. The measurements also demonstrate that the ocean’s pH decreases. Data from near Hawaii show that the pH has decreased by about 0.05 units from 1988 to 2011. Global estimates suggest a decrease by 0.2 units from preindustrial times. This corresponds to a ~30 % increase in hydrogen ions.
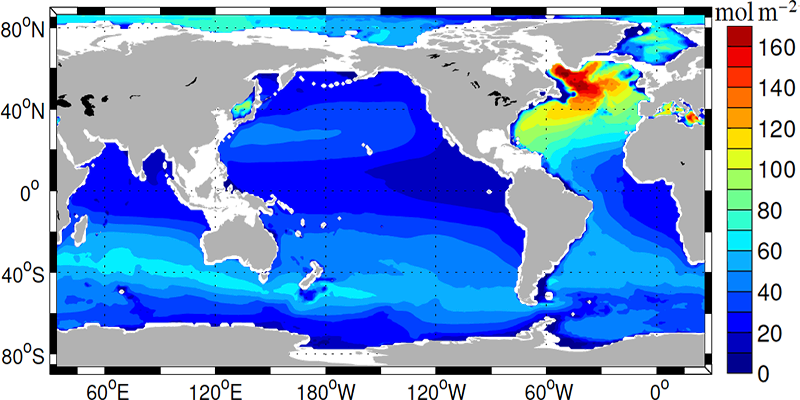
The penetration of anthropogenic carbon into the ocean is largest in the North Atlantic, at mid-latitudes in the Southern Ocean and in the subtropical North Pacific (Fig. 12). As we will see below these are regions of convergence, subduction, or deep water formation.
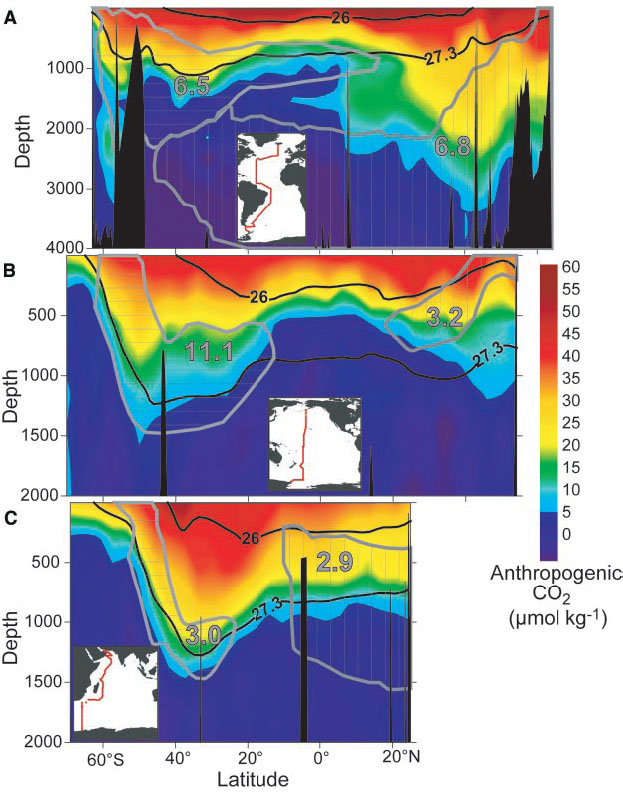
Anthropogenic CO2 enters the ocean at the surface. Therefore most anthropogenic carbon is in the surface layers (Fig. 13). However, measurable amounts have penetrated most of the upper kilometer of the ocean. In some regions such as the North Atlantic and in the Southern Ocean anthropogenic carbon has entered levels below 2 km depth. These are regions in the ocean where surface waters sink to great depths taking anthropogenic carbon with them.
Calcifying organisms such as corals, coccolithophores, foraminifera, and pteropods build shells and other body parts out of calcium carbonate (CaCO3) by using calcium Ca2+ and carbonate CO32- ions (Fig. 14).
Decreasing carbonate ion concentrations and pH will lower the saturation state of calcium carbonate, which will make it more difficult for organisms to build calcium carbonate shells. It will also more easily dissolve existing calcium carbonate. Many scientists are concerned that the currently ongoing changes in the carbonate chemistry of the ocean and those expected for the future, in case of continued anthropogenic carbon emissions, may have adverse consequences for the ocean’s ecosystem. The rates of change are likely much larger than anything experienced in the last millions of years, with unknown risks.
Experiments show that increased CO2 or decreased pH can lead to malformed or partially dissolved coccoliths or pteropod shells. However, ocean acidification research is still in its infancy and consequences for many species and ecosystems are currently not known.
Questions
- What are Earth’s three rapidly exchanging carbon reservoirs?
- Compare anthropogenic carbon emissions from the burning of fossil fuels with the natural carbon emissions from all volcanoes on Earth. How much different is one compared with the other?
- How much carbon does the average American emit per year?
- We can visualize this amount by calculating the corresponding volume of CO2. For this calculation we need to know that density ρ = M/V is mass per volume. We also need to know the density of CO2, which is ρCO2 = 2 kg/m3, and we need to consider that one gram of carbon equals 3.7 grams of CO2: 1 gC = 3.7 gCO2. (That is because carbon has an atomic mass of 12, oxygen has an atomic mass of 18 and thus CO2 has an atomic mass of 12+2×16 = 44. This makes carbon dioxide 44/12 = 3.7 times heavier than carbon.)
- With the above information you should be able to calculate the volume V of CO2 emitted by the average American.
- If this volume V = L3 is a cube, what is the length L of one of its sides?
- How much carbon does the average American emit in a day?
- You can visualize this amount as a volume of CO2 or in bags of charcoal. A large bag sold in the US is about 20 pounds. You can assume charcoal is 100% carbon.
Videos
References
Canadell, J.G., P.M.S. Monteiro, M.H. Costa, L. Cotrim da Cunha, P.M. Cox, A.V. Eliseev, S. Henson, M. Ishii, S. Jaccard, C. Koven, A. Lohila, P.K. Patra, S. Piao, J. Rogelj, S. Syampungani, S. Zaehle, and K. Zickfeld, 2021: Global Carbon and other Biogeochemical Cycles and Feedbacks. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Masson-Delmotte, V., P. Zhai, A. Pirani, S.L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M.I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J.B.R. Matthews, T.K. Maycock, T. Waterfield, O. Yelekçi, R. Yu, and B. Zhou (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp. 673–816, doi:10.1017/9781009157896.007.
Ciais, P., C. Sabine, G. Bala, L. Bopp, V. Brovkin, J. Canadell, A. Chhabra, R. DeFries, J. Galloway, M. Heimann, C. Jones, C. Le Quéré, R.B. Myneni, S. Piao and P. Thornton, 2013: Carbon and Other Biogeochemical Cycles. In: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Stocker, T.F., D. Qin, G.-K. Plattner, M. Tignor, S.K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex and P.M. Midgley (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA.
Khatiwala, S., et al. (2013), Global ocean storage of anthropogenic carbon, Biogeosciences, 10(4), 2169-2191, doi:10.5194/bg-10-2169-2013.
Friedlingstein, P., et al. (2022), Global Carbon Budget 2022, Earth Syst. Sci. Data, 14, 4811–4900, doi: 10.5194/essd-14-4811-2022.
Zeebe, R. E., and D. A. Wolf-Gladrow (2001), CO2 in Seawater: Equilibrium, Kinetics, Isotopes, Elsevier, Amsterdam.
Additional Material
Uptake of something. E.g. a photon of electromagnetic radiation is absorbed by a molecule. Earth’s atmosphere absorbs most infrared radiation from the surface.
The removal of carbon from the surface and sequestration in the deep ocean by marine biota. Phytoplankton take up carbon during photosynthesis at the sunlit surface. They are eaten by zooplankton and the organic matter is transferred through the food web to higher trophic levels. Some of the organic matter sinks to depths, where it is remineralized by bacteria.





