9 Methods for SDS-PAGE
To prepare the crude extract samples for gel electrophoresis, first obtain the 250 µL cell pellets that were stored at -20 °C. Resuspend the pellets in 50 µL of distilled H2O—pipet up and down and vortex to get the entire cell pellet resuspended. Then add 50 µL of 2X SDS dye. Make sure that the SDS dye is completely dissolved before adding to the samples, as SDS precipitates out of solution at low temperatures. Heat samples to 100 °C for about 5-10 minutes, vortexing as necessary to ensure complete dissolution. Centrifuge the samples at >10,000 rcf for 5 minutes. After the gel has been prepared as outlined in the Instruction Protocol, 20 µL of the supernatant can be placed in each well of the polyacrylamide gel. Remember to add 5 µL of molecular weight markers to one well.
To run the gel, follow the Bio-Rad Protean II Instruction Protocol. Once the dye front has reached the bottom of the gel, turn off the voltage and remove the gel from the glass plates. Place the gel in Coomassie Brilliant Blue G-250 staining solution in a plastic container and rock for several hours (or follow Advice below). The staining solution can then be poured off and destain poured onto the gel, followed by additional rocking. The destain can be poured off into the designated waste container in the fume hood and replaced several times until the bands can be clearly visualized against the background of the gel.
- ADVICE: To speed up the staining process, since the staining steps function on electrostatic attraction and diffusion, the gel (in either stain or destain) can be heated in the microwave in the fume hood to boiling (about 20-30 seconds) before placing on the rocker for 10 minutes. Be sure to wear protective eyewear and gloves! Do not leave in destain longer than overnight.
Necessary Materials for Talon-resin Protein Purification
Purification Buffer Preparation and Components
- pH calibration buffers
- 5 N HCl and 8 N NaOH
- Imidazole
- NaCl
- NaH2PO4, Na2HPO4, H3PO4 Na3PO4 salts
Suggested Resources and Protocols
- Use of polyacrylamide gels: The following resources contain useful information for protein gel electrophoresis theory and practice. See page 15 for locations of these resources:
Bio-Rad Mini Protean Tetra Cell handbook (in particular Section 4.2 contains protocols for making stock solutions for discontinuous Laemmli SDS-PAGE gels and buffers).
Voet and Voet
Sambrook’s Molecular Cloning: A Laboratory Manual
- Protein purification buffer preparation: The manual for “TALON Metal Affinity Resins” is posted on the course Canvas site.
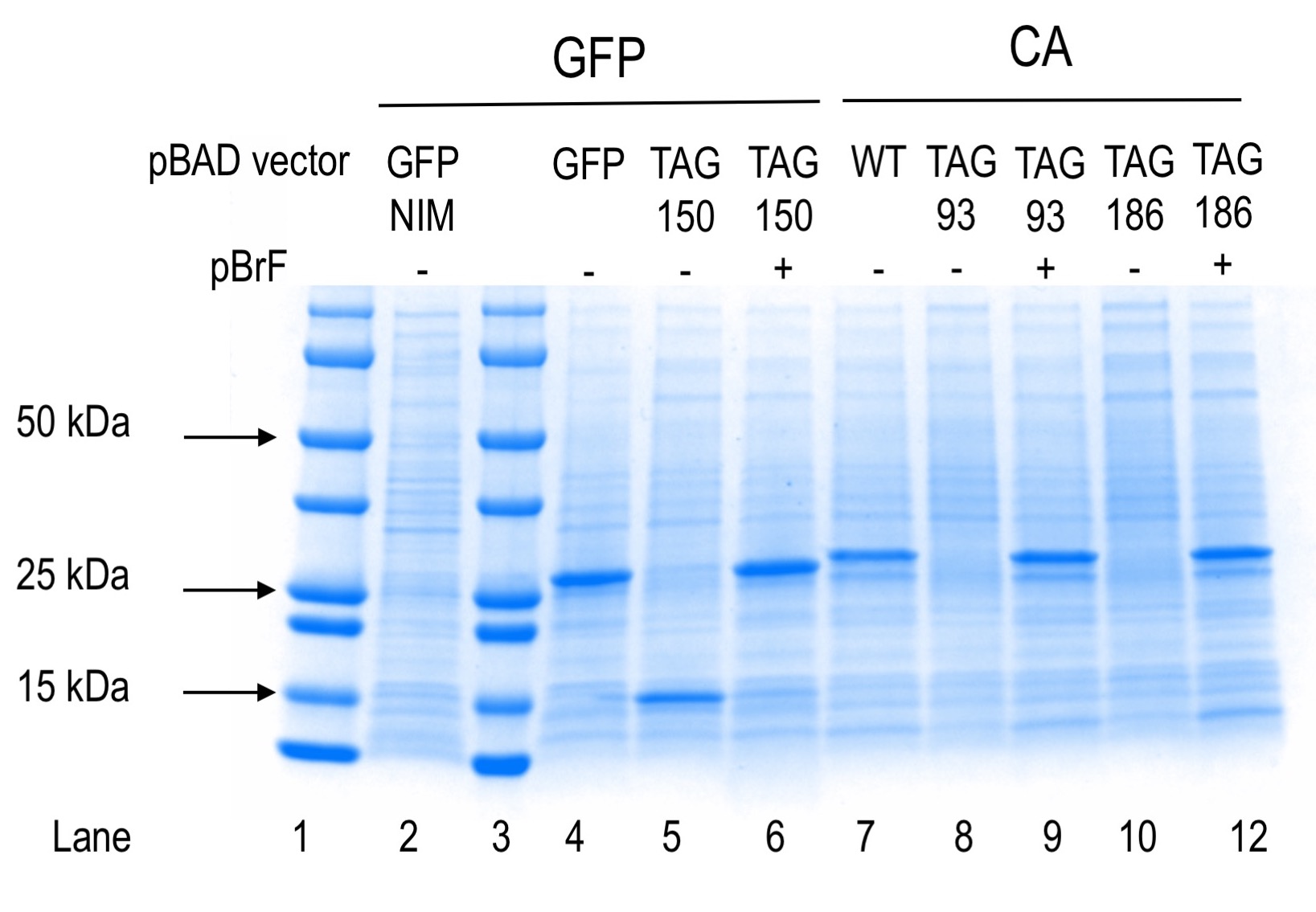
The molecular weight of proteins GFP and CA are 27 and 30 kDa, respectively.
Positive and negative controls for protein production separated by SDS-PAGE and Coomassie stained are shown. Lanes 1 and 3 contain precision plus pre-stained markers (Bio-Rad). Lane 2 shows protein expression from the plasmid indicated for cells grown in non-inducing medium (NIM). Lanes 4-12 show protein expression results from the plasmids indicated in the absence or presence of para-bromophenylalanine (pBrF). GFP150 truncation product is visible in lane 5 at an estimated molecular weight of 15 kDa.